ASD: safe and responsible disinfection by Devea
What is Airborne Surface Disinfection (ASD)?
Surface Disinfection can be either manual or airborne. Airborne Surface Disinfection (ASD) techniques bring three key advantages:
- They are reproducible
- ASD doesn’t require a human presence
- These techniques can uniformly reach all the surfaces of the volume to be treated, including inaccessible areas
The disinfectant or biocide – hydrogen peroxide (H2O2) – is sprayed as a non-wetting micro-drop mist. Once the area to be treated is saturated, it settles on the surfaces and instantly kills bacteria, spores, viruses and yeasts. Hydrogen peroxide converts completely into H2O and O2 – our biocides are therefore 100% biodegradable. As there is no residue, the biocide has no residual effect. Airborne Surface Disinfection (ASD) therefore ponctually decontaminates an area without any residue.

ASD: A clear regulatory framework
The regulation requires efficacy tests to validate two important criteria: fog homogeneous diffusion and efficacy in biocharge reduction.
The efficacy of a diffusion can be evaluated in a simple and relatively fast way. Chemical and/or biological indicators are used to guarantee the efficiency of the biodecontamination and the safety of all users. The disinfection protocols designed with Devea are approved by the customers according to their specifications: reduction of microbial populations by 4-log10 (99.99%), 5-log10 (99.999%) or 6-log10 (99.9999%).
The ASD device/product pair must be approved (requirements of the EN 17272 standards of 2020). Disinfectants must not only comply with the BPR (Biocidal Products Regulation) Reg (EU) 528/2012, but also be registered and authorized for marketing. Devea is the only French ASD company to be granted a Marketing Authorization for its Phileas® solutions (MA FR-2019-0071). This Marketing Authorization validates the diffuser / biocide couple and the achieved results.
The key steps of the biodecontamination process
The validation of the ASD guarantees the restoration of the treated areas to a qualified state. This method of disinfection is part of a complete hygiene process called decontamination* with two main steps: cleaning* then disinfection* (see lexicon below). The cleaning phase is crucial as it removes organic and particulate residues and other chemical contaminants. Only a cleaned surface can be disinfected!
MicroDrop Technology: centrifugation for optimal results
During Airborne Surface Disinfection, the liquid disinfectant is transformed into a mist and then into vapour to penetrate and kill the microbial cells. The challenge is to have the finest drops possible for a covering mist that reaches all surfaces and allows rapid evaporation.
Devea is the only ASD player to provide micro-droplets of 5 to 10μm thanks to centrifugation, a simple yet robust technology patented by Devea. This MicroDrop Technology produces an extremely fine and homogeneous non-wetting mist. It consumes on average 7 times less disinfectant than a manual process and 4 to 5 times less disinfectant than a vapor-based process. The recovery time of the treated area is reduced accordingly.
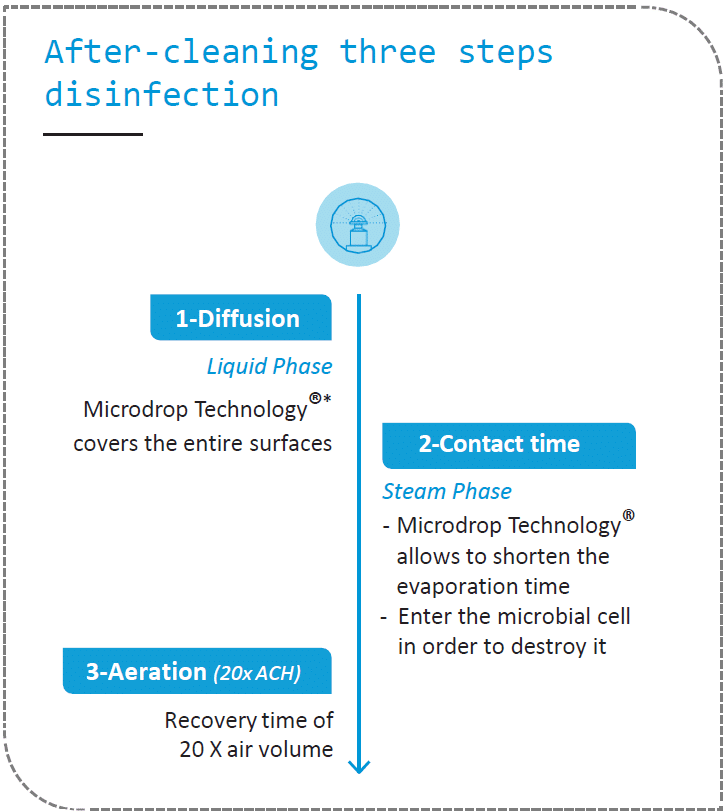
MicroDrop Technology: combining simplicity & efficacy
Setting up an ASD by centrifugation is quick and easy regardless of the temperature and humidity of the room. There is no need to seal doors and windows. Micro-droplets spray is homogeneous, does not wet surfaces and does not require additional fans in the room. The recovery time is shorter due to the low doses of biocide and the size of the droplets. Lastly, O2SAFE® and PHILEASAFE® have a low concentration of H2O2 (<8%). They are therefore non-corrosive, compatible with all laboratory materials and spaces to be disinfected. Transport is not classified as dangerous.




*Cleaning – A process for removing contamination e.g. product residues and disinfectant residues.
*Decontamination – The overall process of removal or reduction of any contaminants (chemical, waste, residue or microorganisms) from an area, object, or person. The method of decontamination used (e.g. cleaning, disinfection, sterilization) should be chosen and validated to achieve a level of cleanliness appropriate to the intended use of the item decontaminated.
*Disinfection – The process by which the reduction of the number of microorganisms is achieved by the irreversible action of a product on their structure or metabolism, to a level judged to be appropriate for a defined purpose.

