Regulation and Disinfection: what is the the regulatory framework for biodecontamination?
NFT 72-281, EN 17-272, BPR 528/2012, European Community conformity, ECHA… What are the norms for ASD devices and biocides? What are the latest applicable versions? Three questions today to Devea’s regulatory expert, specialized in Biocides Regulation EU 528/2012, to help you understand the context.
Devea, French specialist in Airborne Surface Disinfection by centrifugation, has been promoting its Phileas® solutions in France and internationally since 2009. Pharmaceutical industry, hospitals, research laboratories, research animal facilities, health, communities, etc
1. First of all, can you remind us of the regulatory framework in which the ASD, Airborne Surface Disinfection operates?

2. Any recent regulatory changes, and if so, which industry sectors are affected?
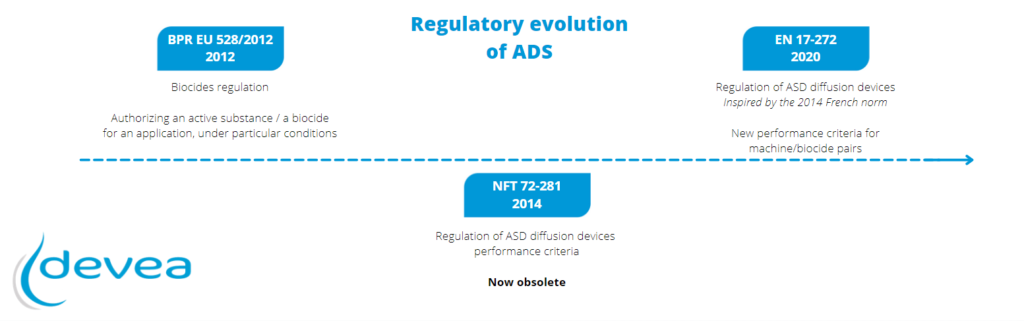
“The French norm NFT 72-281, the last version of which dated from 2014, is now outdated. It largely inspired the European standard EN 17-272 which is currently in force for any request for authorization which would be submitted since its date of publication (April 2020).
Two points in particular have been developed in EN 17-272 compared to NFT 72-281:
a) A distribution test to validate the homogeneity of the diffusion of biocide fog, with 8 biological indicators distributed in the corners.
b) Populations of microorganisms and a log reduction in these populations variable according to the target industries.
Specifically for the pharmaceutical industry, the production of sterile drugs, ruled by appendix I of ‘Good Manufacturing Practices’, is evolving towards the promotion of barrier technologies, and a limitation of human interventions in grade A areas. Bioburden control, minimization of aseptic risks and a more structured contamination control strategy are the pillars of this new version 12. This appendix I describes the need to biodecontaminate the most critical areas in sterile production, and to validate the effectiveness of cleaning and disinfection. The ASD is therefore a key step to qualify these areas, and choosing the right ASD partner can make all the difference! High-performing equipment, expert support and time saving in establishing effective protocols, simple yet complete traceability, service provision, maintenance and adapted services, etc.”
3. At the start of the year, should we expect any new changes for 2022?
“The official publication of this version 12 of appendix I of the GMP is expected for the spring, even if the text has been finalized since December 2020. A first draft of version 13 is already circulating…”


